| Structure/Class |
|
| Pharmacodynamics |
- In CNS, ethanol affects many different receptors
- Increased GABA activity at the GABA-A receptor
- Decreased glutamate activity at the NMDA receptor
- Also affects the Na/K ATPase, phospholipase C and other ion channels.
- Organ system effects (acute)
- CNS
- Disinhibition, sedation, anxiolysis
- At higher doses – coma, respiratory depression and death
- CVS
- Decreased myocardial contractility
- Smooth muscle
- Vasodilation, probably due to acetaldehyde
- Organ system effects (chronic)
- GIT and liver
- Fatty liver disease à alcoholic hepatitis à cirrhosis/liver failure
- Chronic pancreatitis
- Gastritis
- Multiple vitamin deficiencies (especially water soluble ones)
- Nervous system
- Tolerance and both physical and psychological dependence.
- Withdrawal syndrome/delirium tremens if acute cessation. DTs are characterized by excitability/agitation and may cause seizures.
- Neurotoxicity – peripheral neuropathy, ataxia, Wernicke-Korsakoff encephalopathy and optic neuropathy
- CVS
- Dilated cardiomyopathy with ventricular hypertrophy and fibrosis
- Arrhythmias, probably due to chronic electrolyte abnormalities
- HTN
- Vascular disease – stroke/MI/PVD
- Haematological
- Iron deficiency anaemia secondary to gastritis
- Folate deficiency – megaloblastic anaemia
|
| Absorption/administration |
- PO
|
| Distribution |
- Well absorbed from GIT. Peak blood levels in 30 minutes.
- Vd is ~0.7L/kg (total body water)
- Ethanol easily passes BBB, and therefore readily enters the CNS.
|
| Metabolism |
- Metabolism follows zero order kinetics
- ~7-10mg/hour typically metabolized by average adult.
- Ethanol is metabolized to acetaldehyde via two major pathways:
- Alcohol dehydrogenase pathway
- Alcohol dehydrogenase found mainly in liver, but small amounts can be found in the brain and stomach.
- Alcohol is converted by alcohol dehydrogenase to acetaldehyde. NAD+ acts as a co-factor, and is converted to NADH.
- Note that in acute toxicity, NADH will accumulate and cause lactic acidosis and hypoglycaemia.
- Microsomal ethanol oxidizing system (MEOS)
- MEOS uses CYP450 enzymes and NADPH as a co-factor.
- It is inducible (and therefore ethanol may induce the metabolism of other drugs)
- The acetaldehyde is then converted to acetate, which is broken down to CO2 and H2O. The enzyme metabolizing this reaction is aldehyde dehydrogenase.
- Note that aldehyde dehydrogenase (and not alcohol dehydrogenase) is inhibited by disulfiram and other drugs (e.g. metronidazole and trimethoprim)
|
| Excretion |
- Urine
- Small amount in lungs (forms the basis for ethanol breathalyzer tests)
|
| Indications |
|
| Contraindications |
|
| Special precautions |
- Be prepared to treat respiratory and CNS complications in acute intoxication
- Respiratory: be prepared for respiratory depression, vomiting and aspiration
- CNS: remember to give thiamine.
- Also give glucose (monitor for hypoglycaemia)
|
| Interactions |
- Enzyme induction leads to increased metabolism of drugs metabolized by the CYP450 system. Especially important in concurrent paracetamol overdose – increased metabolism of paracetamol to its toxic byproducts may increase hepatotoxicity.
- Additive effects with other CNS depressants.
|
| Adverse events |
- As mentioned above
|
| Dosing/administration |
|
| Toxicology |
Methanol
- Found in “canned heat” and windshield washing products
- The toxicity of methanol is due to the metabolic byproducts of formaldehyde and formic acid
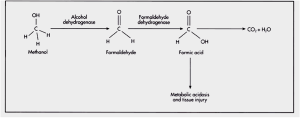
- Symptoms (note that because symptoms are due to metabolic byproducts, they are only seen ~6-30 hours post ingestion)
- Non-specific early signs (intoxication, gastritis, high osmolar gap)
- Characteristic visual disturbance – “like being in a snowstorm”
- May progress to bradycardia, coma and seizures.
- Death is due to respiratory arrest
- Treatment
- Good supportive care
- Suppression of metabolism – fomepizole and IV ethanol are temporizing measures
- Removal of toxin – haemodialysis
- Alkalinisation in order to counteract metabolic acidosis (Na bicarbonate)
- Folic acid and folinic acid may be useful adjuncts (but does not directly improve patient’s clinical state)
Ethylene glycol
- Found in antifreeze/industrial solvent
- Toxicity comes from its toxic byproducts (aldehydes and oxalate crystals)
- Symptoms occur in three stages:
- First few hours: CNS excitation, then CNS depression
- Severe metabolic acidosis
- Renal failure, due to oxalate crystals.
- Features suggestive of ethylene glycol toxicity are HAGMA, high osmolar gap and oxalate crystals in urine. There is no visual disturbance.
- Treatment
- As with methanol toxicity: good supportive care, fomepizole, IV ethanol and haemodialysis.
|
| Withdrawal syndrome |
|
| Special notes |
|
