Defence of tonicity of ECF
- Mainly the job of thirst and vasopressin secreting mechanisms
- Body osmolality is proportional to Na+, K+ and divided by total body water
- Normal osm 280-295mOsm/kg
- Hypertonic –> more vassopressin release. Osmoreceptors in hypothalamus outside BBB.
- 3 kinds of Vasopressin receptors. Main one is V2 is cGMP mediated –> aquaporin insertion in collecting ducts
- V1a –> vasoconstrictor effect
- Vasopressin – posterior pituitary
- Secretion- osm >285, standing, haemorrhage/decrease ECF(via low pressure and High pressure sensors), hypotension, pain, emotion, stress, nausea, vomiting, clofibrate, carbamazepine, Angiotensin II
- EtOH decreases vasopressin secretion
- Volume stimuli overrides osmotic stimuli
- Diabetes insipidus = ADH deficiency
- SIADH–> dilutional hyponatraemia
Defence of volume.
- Amount of Na+ in ECF is the most important determinant of ECF volume
- Renin – angiotensin – aldosterone system –> vasoconstriction + Na and water retention
- Angiotensin II is key for volume control
- Too much volume –> BNP and ANP –> natriuresis and diuresis
- Renin secretion
- Renal nerves firing
- Increased catecholamines
- Prostaglandins
- Renin secretion inhibition
- Angiotensin II
- Vasoppressin
- Increased Na and cl absorption at macula densa
- Increased afferent arteriolar pressure
Defence of H+ concentration (Acid-base balance).
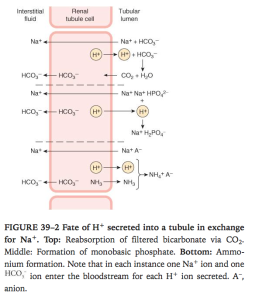
Ganong’s Review of Medical Physiology, 24th Edition
- In PCT this is via secondary active counter transport of Na/H at apical membrane
- H+ is formed from H2CO3 so for each H+ secreted a Na and HCO3 diffuse into interstitum. Inhibitng carbonic anhydrase inhibits H+ secretion and down stream reactions
- in PCT most of the secreted H+ reacts with HCO3– to form H2CO3. The H2CO3 breaks down to form CO2 and H2O. In the proximal (but not in thedistal) tubule, there is carbonic anhydrase in the brush border of the cells; this facilitates the formation of CO2 and H2O in the tubular fluid. The CO2, which diffuses readily across all biological membranes, enters the tubular cells, where it adds to the pool of CO2 available to form H2CO3. Because most of the H+ is removed from the tubule, the pH of the fluid is changed very little. This is the mechanism by which HCO3–is reabsorbed;for each mole of HCO3–removed from the tubular fluid,1 mol of HCO3–diffuses from tubular cells into the blood, even though it is not the same mole that disappeared from the tubular fluid.
- In DCT and collecting ducts its via H+ ATPase, independent of Na. aldosterone promotes this pump.
- More PCO2 –> more H2CO3–> more acid secretion
- More K –> more intracellular acidosis –> more acid secretion
- More Aldosterone –> more H+ and K+ secretion.
- H+ reacts with Phosphate in DCT and CD. Reacts with NH3 in proximal and distal tubules.
- NH3 is non ionic so easily diffuses into tubular fluid. NH3:NH4 is 1: 100. The NH3 reacts with H+ to make NH4+ quickly which is trapped in lumen and NH3 concentration gradient is still maintained so more NH3 keeps diffusing into tubular fluid.
- H+ secretion has little effect on pH due to it being converted to CO2 and H2O. Even a small amount of H+ secreted distally has a bigger effect on urine acidity than the large amounts in proximal tubule.
- Bi carb secretion
- HCO3–reabsorption is decreased by an unknown mechanism whenthe extracellular fluid (ECF) volume is expanded. When the plasma HCO3–concentration is low, all the filtered HCO3–is reabsorbed; but when the plasma HCO3-concentration is high, HCO3 appears in the urine and the urine becomes alkaline. Conversely, when the plasma HCO3 – falls below about 26 mEq/L, the value at which all the secreted H+ is being used to reabsorb HCO3 –, more H+ becomes available to combine with other buffer anions. Therefore, the lower the plasma HCO3– concentration drops, the more acidic the urine becomes and the greater its NH4+ content.
Definition of pH.
- Negative log10 of H+ concentration
Buffers.
- The maximal H+ gradient against which the transport mechanisms can secrete in humans corresponds to a urine pH of about 4.5, this is the limiting pH. This is usually reached in collecting ducts. It would be reached much earlier if it wasnt for the buffers in the tubular fluid remove free H + , permitting more acid to be secreted. These are the reactions with HCO3– to form CO 2 and H 2 O, with HPO42–to form H2PO4–, and with NH3to form NH4+.
- Blood buffers – HCO3, Protein, Hb
- Intracellular buffers – HPO4, protein
Acid-base disturbances.
- Resp acidosis –> high PCO2 –> high HCO3- –> increase H+ secretion and HCO3 reabsorption
- Resp alkalosis –> low PCO2 –> decrease H+ secretion and HCO3 reabsorption
- Metabolic acidosis
- High anion gap – ketoacidosis, lactic acidosis, toxins(asprin, ethelyne glycol, cyanide, isoniazid), renal failure
- Normal anion gap – GIT losses, renal loss of bicarb, renal dysfunction(RTA), azetozolamide, TPN, Addisons
- Compensated by :Increase resp rate and Increase renal secretion of H+
- Metabolic alkalosis
- Form adding base or removing acid
- Resp suppression
- Decrease renal reabsorption of HCO3
- The formula expressing the expected C02 level in a metabolic acidosis is CO2=(1.5 x HCO3) +8
Basic ABG interpretation
- Anion gap – difference between the measured cations minus anions
- (Na+K) – (Cl+ HCO3)
- Daily practice we ignore K
- Normal is 3-11
- Standard bicarbonate.
- What the plasma bicarbonate would be once the respiratory component is eliminated
- Buffer base.
- The buffer base is equal to the total number of buffer anions (principally Prot–, HCO3–, and Hb–) that can accept hydrogen ions in the blood.
- Base excess.
- The amount of acid or base that would restore 1 L of blood to normal acid–base composition at a PCO2 of 40mm Hg.
Viva questions:
- Tell me about renal H+/HCO3- handling
- Tell me about renal water handling.
- What factors operate to maintain normal tonicity / osmolality ?
- What factors operate to maintain normal ECF volume ?
- Tell me about the main buffers in the body
- How does the body respond to an acid load
- Tell me about metabolic acidosis and compensatory mechanisms
- Tell me about metabolic alkalosis and compensatory mechanisms
- Tell me about respiratory acidosis and compensatory mechanisms
- Tell me about respiratory alkalosis and compensatory mechanisms
