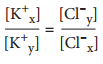Body fluid compartments
(particularly volumes and how they are measured. Rules of 3 is quite helpful).
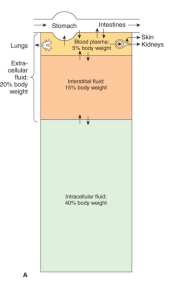
- Total Body Fluid 2/3 (60% of Total Body weight)
- ECF – 1/3 (20% of Total Body weight)
- Intravascualar/blood plasma 1/4 (5% of Total Body weight)
- Total blood volume = plasma + cell contents of blood
- Interstitial 3/4
- Intravascualar/blood plasma 1/4 (5% of Total Body weight)
- ICF – 2/3 (40% of Total Body weight)
In Percentage of body wt – ICF 40%, interstial 15%, plasma 5%
Contents – ECF is high in Na+Cl-, ICF is high in K+, Mg+, proteins and phosphates
Transport across cell membranes
- Passive diffusion down electrochemical gradient
- Active transport eg Na+ / K+ ATP-ase which uses energy to transport ions against the gradient
- (3Na out and 2K in), alpha and beta subunits, spans whole membrane
- Secondary active transport when substances are transported down a gradient set up by a separate active transport eg glucose in bowel.
- Co-transport
- Counter-transport
- Ion channels – voltage gated or ligand gated
- Aquaporins
- Endocytois/exocytosis
Membrane potential.
The difference in electrical charge between the inside and outside of the biological cell. With respect to the exterior the typical values range from -40mV to -60mV
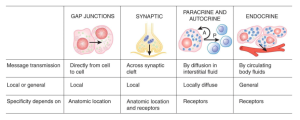
Fundamentals of Intercellular Communication and 2nd messengers
Second messenger system
Extracellular ligand binds a surface receptor –> morphological or chemical change in the intra cellular part of the receptor which will activate an enzyme, G Protein activation, ion channel activation, direct transcription etc –> increase in intracellular cAMP, Ca2+ etc –> secondary effect eg increased protein production
Less important:
- Read “Functional morphology of the cell” once only or not at all.
- Ditto with the DNA / RNA / meiosis / mitosis stuff.
Definitions:
Transcellular fluid.
The proportion of body water contained in epithelium lined spaces
Moles vs. Osmoles.
- Moles = weight in grams of 6×10^23 of a substance = molecular wt in grams.
- Osmoles = molecular weight/number of free moving particles each molecule liberates in solution
- IF a substance dissociates into two 1mole = 2osmoles in an ideal solution.
Equivalents.
1 mole of an ionized substance divided by its valence(charge)
Osmolarity vs. Osmolality.
- The osmolarity is the number of osmoles per liter of solution (eg. plasma), whereas the osmolality is the number of osmoles per kilogram of solvent.
- 1 mol of ideal solution depresses freezing point by 1.86oC
- Normal plasma osmolarity is 290mOsm/L
pH.
- pH = -log([H+])
- Water at 25oC is 7 means H+ = OH-
- Normal human plasma pH 7.35-7.45, different in various body environments to maximise protein/enzyme functions
Buffer.
A substance that can bind or release H+ in order to maintain a stable pH eg H2CO3, phosphates , proteins
Diffusion.
Process where a gas or substance in solution spreads into the total volume available to it by the random movement of its particles. In areas of higher concentration there are more particles moving to areas of lower concentration = net flux. Time to equilibrium is proportional to square of diffusion distance. The magnitude of diffusion is proportional to concentration gradient and cross section area.
Solvent drag.
The influence exerted by a flow of solvent through a membrane on the simultaneous movement of a solute through the membrane
Osmosis
Diffusion of solvent molecules into an area of higher solute concentration but is impermeable to the solute
Osmotic pressure.
Is the pressure necessary to prevent solvent migration. It is proportional to number of particles per volume in solution. Only acts when the solution comes into contact with another across a membrane permeable only to solvent.
Tonicity
Osmolality of a substance related to plasma osmolality.
Donnan effect.
When an ion on one side of a membrane is impermeable it affects the movements of the permeable ions too eg an impermeable anion will hinder movement of a cation while encourage movement of an anion across the membrane filtration.
Fick’s law
“The magnitude of the diffusing tendency from one region to another is directly proportional to the cross-sectional area across which diffusion is taking place and the concentration gradient, or chemical gradient, which is the difference in concentration of the diffusing substance divided by the thickness of the boundary”
Oncotic pressure.
A form of osmotic pressure exerted by proteins in blood vessels that usually tend to pull water into the circulatory system.
Viva questions:
Describe the body’s fluid compartments and their volumes.
How is TBW / ICF volume / Interstitial fluid volume / ECF volume / plasma volume / red cell mass measured ?
In what ways may ions and other small molecules be transported across cell membranes ?
Describe the Na+ / K+ ATP-ase pump.
What are the net ionic transport results of it’s operation ?
What is Fick’s law ?
How can you calculate osmolality ? ( And what is the normal value ?)
How is the resting membrane potential of a neuron created ?
What is a second messenger ?
What second messenger systems can you describe ?